Multiple Choice
Drawing (1) shows a system in which an equilibrium exists between dissolved and undissolved gas particles at P = 1 atm.According to Henry's law,if the pressure is increased to 2 atm and equilibrium is restored,which drawing (2) -(5) best represents the equilibrium at 2 atm? 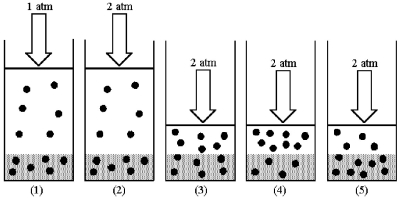
A) drawing (2)
B) drawing (3)
C) drawing (4)
D) drawing (5)
Correct Answer:

Verified
Correct Answer:
Verified
Q57: Arrows in the energy diagram below represent
Q67: In most liquid solutions,the component present in
Q68: For the process of dissolving a solid
Q69: Which ion-dipole interaction results in the larger
Q70: A KCl solution is prepared by dissolving
Q71: Aqueous solutions of 30% (by weight)hydrogen peroxide,H<sub>2</sub>O<sub>2</sub>,are
Q73: Calculate the freezing point of a solution
Q75: In general,as the temperature increases,the solubility of
Q76: The change in the Gibbs free energy
Q77: Which of the following mixtures have components