Multiple Choice
The following pictures represent solutions at various stages in the titration of a weak diprotic acid H2A with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A2- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity) . 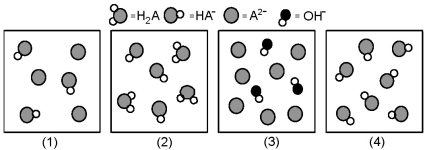
-Which picture represents the system at the first equivalence point?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q4: What is the pH of the resulting
Q5: What is the pH of a buffer
Q7: State whether the solubility of Mg(OH)<sub>2</sub> will
Q8: The following pictures represent solutions that contain
Q10: What is the pH at the equivalence
Q11: What is the [CH<sub>3</sub>CO<sub>2</sub><sup>-</sup>]/[CH<sub>3</sub>CO<sub>2</sub>H] ratio necessary to
Q12: Which is a net ionic equation for
Q13: Calculate the K<sub>sp</sub> for silver sulfate if
Q14: The balanced net ionic equation for the
Q122: The following pictures represent solutions that contain