Multiple Choice
When suspected drunk drivers are tested with a Breathalyzer,the alcohol (ethanol) in the exhaled breath is oxidized to acetic acid with an acidic solution of potassium dichromate: 3 CH3CH2OH(aq) + 2 Cr2O7-2 (aq) + 16 H+(aq) 3CH3CO2H(aq) + 4Cr+3(aq) + 11H2O (l)
What is the value of E for the reaction when the concentrations of ethanol,acetic acid,Cr2O7-2,and Cr+3,are 1.0M and the pH is 4.00? (Eº for this cell is 1.30V and the temperature is 298K) .
Equations to solve this problem are below.
Ε = ε° - 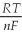 lnQ
lnQ
Ε = 1.30 - 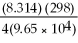 ln
ln 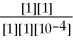
A) 1.62 V
B) 1.30 V
C) 0.98 V
D) 1.24 V
Correct Answer:

Verified
Correct Answer:
Verified
Q78: For the galvanic cell reaction,expressed below using
Q123: Consider the following galvanic cell. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg"
Q125: Based on the balanced chemical equation shown
Q126: Using the following standard reduction potentials, Fe<sup>3+</sup>(aq)+
Q127: What is the relationship between the standard
Q129: What is the standard cell potential for
Q130: A galvanic cell employs the reaction Ni<sup>2+</sup>(aq)+
Q131: Which statement concerning overvoltage is false?<br>A)Overvoltage is
Q132: The cell reaction for a lead storage
Q133: Consider the following standard reduction potentials, Al<sup>3+</sup>(aq)+