Multiple Choice
What is the relationship between the standard cell potentials,E°,for the following two galvanic cell reactions? I.3 Cu2+(aq) + 2 Al(s) → 3 Cu(s) + 2 Al3+(aq)
II.30 Cu2+(aq) + 22 Al(s) → 30 Cu(s) + 22 Al3+(aq)
A) E°(I) = E°(II)
B) E°(I) = 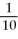
E°(II)
C) E°(I) = 10E°(II)
D) E°(I) = E°(II) 10
Correct Answer:

Verified
Correct Answer:
Verified
Q78: For the galvanic cell reaction,expressed below using
Q122: In the galvanic cell represented by the
Q123: Consider the following galvanic cell. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg"
Q125: Based on the balanced chemical equation shown
Q126: Using the following standard reduction potentials, Fe<sup>3+</sup>(aq)+
Q128: When suspected drunk drivers are tested with
Q129: What is the standard cell potential for
Q130: A galvanic cell employs the reaction Ni<sup>2+</sup>(aq)+
Q131: Which statement concerning overvoltage is false?<br>A)Overvoltage is
Q132: The cell reaction for a lead storage