Multiple Choice
Consider the following galvanic cell. 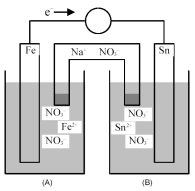
-What is the quantitative change in the cell voltage on increasing the ion concentration in the anode compartment by a factor of 10?
A) +0.06 V
B) +0.03 V
C) -0.03 V
D) -0.06 V
Correct Answer:

Verified
Correct Answer:
Verified
Q78: For the galvanic cell reaction,expressed below using
Q118: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -For the galvanic
Q119: Shown below are the reactions occurring in
Q120: A particular 12V battery is based on
Q121: Fuel cells<br>A)produce carbon dioxide and hydrogen.<br>B)emit sulfur
Q122: In the galvanic cell represented by the
Q125: Based on the balanced chemical equation shown
Q126: Using the following standard reduction potentials, Fe<sup>3+</sup>(aq)+
Q127: What is the relationship between the standard
Q128: When suspected drunk drivers are tested with