Multiple Choice
In a reversible heat engine, one mole of an ideal gas is carried through a circular cycle beginning and ending at point A as shown in the figure. Which one of the following statements concerning this system is false? 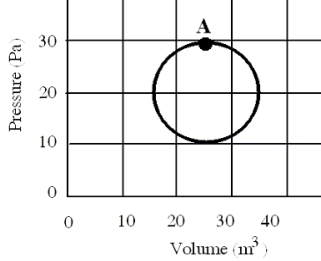
A) The entropy must increase in one cycle.
B) The heat added in one cycle must be 314 J.
C) The work done in completing one cycle is 314 J.
D) The change in internal energy for one cycle is zero joules.
E) The internal energy for this system is dependent on its state.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Two moles of an ideal gas have
Q9: 5.00 kg of liquid water is heated
Q10: Which one of the following situations is
Q13: Two moles of an ideal gas have
Q14: A thermally isolated sample of an ideal
Q16: Enclosed beneath the moveable piston in the
Q17: A quantity of carbon monoxide gas is
Q20: A Carnot engine operates between hot and
Q67: What change in temperature occurs when 1600
Q77: An ideal monatomic gas expands isobarically from