Multiple Choice
A thermally isolated sample of an ideal gas at a fixed temperature is confined to one half of a container by an impermeable membrane. The other half of the container is evacuated. The membrane is then pierced and the gas is allowed to expand freely and to double its volume as shown. Which one of the following statements is true concerning this situation? 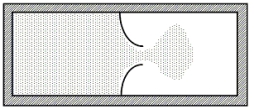
A) The process is reversible.
B) This is an isothermal process.
C) The entropy of the gas decreases.
D) The internal energy of the gas must decrease.
E) The temperature of the gas decreases to one-half of its original value.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: 5.00 kg of liquid water is heated
Q10: Which one of the following situations is
Q12: In a reversible heat engine, one mole
Q13: Two moles of an ideal gas have
Q16: Enclosed beneath the moveable piston in the
Q17: A quantity of carbon monoxide gas is
Q18: An ideal monatomic gas expands isobarically from
Q20: A Carnot engine operates between hot and
Q53: Which one of the following statements best
Q77: An ideal monatomic gas expands isobarically from