Multiple Choice
An ideal monatomic gas expands isothermally from state A to state B. The gas then cools at constant volume to state C. The gas is then compressed isobarically to D before it is heated until it returns to state A. 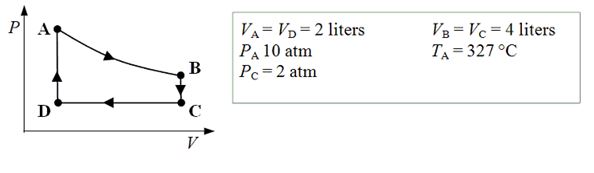
-What is the internal energy of the gas at point B?
A) 1 × 103 J
B) 2 × 103 J
C) 3 × 103 J
D) 4 × 103 J
E) 5 × 103 J
Correct Answer:

Verified
Correct Answer:
Verified
Q13: An ideal monatomic gas originally in state
Q22: 5.00 kg of liquid water is heated
Q25: 5.00 kg of liquid water is heated
Q28: In an isothermal and reversible process, 945
Q29: An ideal monatomic gas expands isothermally from
Q29: Two moles of a confined ideal monatomic
Q30: The ratio of the molar specific
Q40: An ideal monatomic gas originally in state
Q48: An ideal monatomic gas expands isothermally from
Q69: A container holding 1.2 kg of water