Multiple Choice
Given the data in the table below,ΔH°rxn for the reaction IF5 (g) + F2 (g) → IF7 (g)
Is ________ kJ. 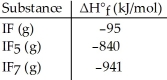
A) 1801
B) -1801
C) 121
D) -121
E) -101
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q48: A 100-watt electric incandescent light bulb consumes
Q49: The value of ΔH° for the reaction
Q50: The temperature of a 15-g sample of
Q51: Given the following reactions N<sub>2</sub> (g)+ O<sub>2</sub>
Q52: A typical fast food meal consists of
Q54: A 26.9 g rock rolls down the
Q55: How many joules of heat are absorbed
Q56: Which of the following is a statement
Q57: The change in the internal energy of
Q58: The value of ΔH° for the following