Multiple Choice
The value of ΔH° for the following reaction is 177.8 kJ.The value of Δ 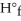 for CaO(s) is ________ kJ/mol. CaCO3 (s) → CaO (s) + CO2 (g)
for CaO(s) is ________ kJ/mol. CaCO3 (s) → CaO (s) + CO2 (g) 
A) -1600
B) -813.4
C) -635.5
D) 813.4
E) 177.8
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q53: Given the data in the table below,ΔH°<sub>rxn</sub>
Q54: A 26.9 g rock rolls down the
Q55: How many joules of heat are absorbed
Q56: Which of the following is a statement
Q57: The change in the internal energy of
Q59: Of the following,ΔH°<sub>f</sub> is not zero for
Q60: The temperature of a 24.3 g sample
Q61: A 22.9 g sample of iron absorbs
Q62: A 5.00-g sample of copper metal at
Q63: What is the kinetic energy of a