Multiple Choice
How many joules of heat are absorbed when the temperature of a 13.9 g sample of CaCO3 (s) increases from 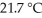 to
to 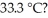 Specific heat of calcium carbonate is
Specific heat of calcium carbonate is 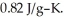
A) 130 J
B) 0.68 J
C) s-130 J
D) -0.68 J
E) 9.5 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q50: The temperature of a 15-g sample of
Q51: Given the following reactions N<sub>2</sub> (g)+ O<sub>2</sub>
Q52: A typical fast food meal consists of
Q53: Given the data in the table below,ΔH°<sub>rxn</sub>
Q54: A 26.9 g rock rolls down the
Q56: Which of the following is a statement
Q57: The change in the internal energy of
Q58: The value of ΔH° for the following
Q59: Of the following,ΔH°<sub>f</sub> is not zero for
Q60: The temperature of a 24.3 g sample