Multiple Choice
Calculate the vapor pressure of a solution made by dissolving 109 grams of glucose (molar mass = 180.2 g/mol) in 920.0 ml of water at 25 °C.The vapor pressure of pure water at 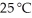 is
is 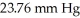 .Assume the density of the solution is 1.00 g/ml.
.Assume the density of the solution is 1.00 g/ml.
A) 0.278 mm Hg
B) 0.605 mm Hg
C) 22.98 mm Hg
D) 23.48 mm Hg
E) 23.76 mm Hg
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The concentration (M)of HCl in a solution
Q2: A solution is prepared by dissolving 15.8
Q3: The vapor pressure of pure water at
Q5: A solution is prepared by dissolving 25.0
Q6: Which component of air is the primary
Q7: What is the mole fraction of HCl
Q8: How many grams of solution are present
Q9: The process of solute particles being surrounded
Q10: The vapor pressure of pure ethanol at
Q11: Calculate the freezing point of a 0.09500