Multiple Choice
The concentration of lead nitrate (Pb(N 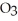
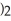 ) in a 0.926 M solution is ________ molal.The density of the solution is 1.202 g/mL.
) in a 0.926 M solution is ________ molal.The density of the solution is 1.202 g/mL.
A) 0.770
B) 2.13
C) 1.03
D) 0.819
E) 0.650
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q25: What is the molality of a 36.1%
Q26: The molarity of urea (MW = 60.0
Q27: A 0.200 m solution of which one
Q28: An aqueous solution of a soluble compound
Q29: Emulsifying agents typically have a hydrophobic end
Q31: What is the molality of a 10.0%
Q32: A solution is prepared by dissolving 2.00
Q33: Which produces the greatest number of ions
Q34: What is the molality of a 24.4%
Q35: The concentration of CO<sub>2</sub> in a soft