Multiple Choice
A solution is prepared by dissolving 2.00 g of glycerin ( 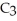
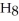
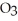 ) in 201 g of ethanol
) in 201 g of ethanol 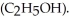 The freezing point of the solution is ________°C.The freezing point of pure ethanol is
The freezing point of the solution is ________°C.The freezing point of pure ethanol is 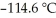 at 1 atm.The molal-freezing-point-depression constant (
at 1 atm.The molal-freezing-point-depression constant ( 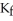 ) for ethanol is
) for ethanol is 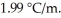 The molar masses of glycerin and of ethanol are 92.1 g/mol and 46.1 g/mol,respectively.
The molar masses of glycerin and of ethanol are 92.1 g/mol and 46.1 g/mol,respectively.
A) -116.6
B) 0.215
C) -112.6
D) -114.4
E) -114.8
Correct Answer:

Verified
Correct Answer:
Verified
Q27: A 0.200 m solution of which one
Q28: An aqueous solution of a soluble compound
Q29: Emulsifying agents typically have a hydrophobic end
Q30: The concentration of lead nitrate (Pb(N <img
Q31: What is the molality of a 10.0%
Q33: Which produces the greatest number of ions
Q34: What is the molality of a 24.4%
Q35: The concentration of CO<sub>2</sub> in a soft
Q36: What is the molality of LiCl in
Q37: What is the molarity of phosphoric acid