Multiple Choice
The elementary reaction 2NO2 (g) → 2NO (g) + O2 (g)
Is second order in 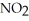 and the rate constant at 660 K is 5.23
and the rate constant at 660 K is 5.23 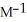
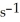 .The reaction half-life at this temperature when
.The reaction half-life at this temperature when 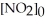 = 0.45 M is ________ s.
= 0.45 M is ________ s.
A) 2.4
B) 7.6
C) 0.19
D) 0.13
E) 0.42
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The half-life of a first-order reaction is
Q3: Rates of reaction can be positive or
Q4: A second-order reaction has a half-life of
Q5: Define activation energy.
Q6: If a rate law is first order
Q7: What is the order of the reaction
Q8: The rate constant of a first-order process
Q9: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt=" decomposes
Q10: The enzyme _ converts nitrogen into ammonia.<br>A)oxygenase<br>B)hydrogenase<br>C)oxidase<br>D)nitroglycerine<br>E)nitrogenase
Q11: Of the following,_ will lower the activation