Multiple Choice
The half-life of a first-order reaction is 13 min.If the initial concentration of reactant is 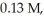 it takes
it takes  for it to decrease to 0.085 M.
for it to decrease to 0.085 M.
A) 12
B) 10.
C) 8.0
D) 11
E) 7.0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: The elementary reaction 2NO<sub>2</sub> (g)→ 2NO (g)+
Q3: Rates of reaction can be positive or
Q4: A second-order reaction has a half-life of
Q5: Define activation energy.
Q6: If a rate law is first order
Q7: What is the order of the reaction
Q8: The rate constant of a first-order process
Q9: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt=" decomposes
Q10: The enzyme _ converts nitrogen into ammonia.<br>A)oxygenase<br>B)hydrogenase<br>C)oxidase<br>D)nitroglycerine<br>E)nitrogenase
Q11: Of the following,_ will lower the activation