Multiple Choice
Calculate the pH of a solution prepared by dissolving 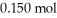 of benzoic acid and
of benzoic acid and 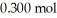 of sodium benzoate in water sufficient to yield
of sodium benzoate in water sufficient to yield 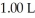 of solution.The Ka of benzoic acid is
of solution.The Ka of benzoic acid is 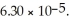
A) 2.516
B) 3.892
C) 4.502
D) 10.158
E) 4.195
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q94: Calculate the pH of a solution that
Q95: Suppose you have just added 500.0 ml
Q96: A 25.0 mL sample of 0.150 M
Q97: Of the substances below,_ will decrease the
Q98: The concentration of fluoride ions in a
Q100: A 25.0 mL sample of 0.723 M
Q101: Why does fluoride treatment render teeth more
Q102: What is the pH of a solution
Q103: A solution of NaF is added dropwise
Q104: What is the solubility (in M)of PbCl<sub>2</sub>