Multiple Choice
The concentration of fluoride ions in a saturated solution of barium fluoride is ________ M.The solubility product constant of BaF2 is 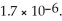
A) 3.8 × 10-4
B) 3.0 × 10-3
C) 1.5 × 10-2
D) 7.5 × 10-3
E) 1.4 × 10-4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q93: The addition of HF and _ to
Q94: Calculate the pH of a solution that
Q95: Suppose you have just added 500.0 ml
Q96: A 25.0 mL sample of 0.150 M
Q97: Of the substances below,_ will decrease the
Q99: Calculate the pH of a solution prepared
Q100: A 25.0 mL sample of 0.723 M
Q101: Why does fluoride treatment render teeth more
Q102: What is the pH of a solution
Q103: A solution of NaF is added dropwise