Multiple Choice
A 25.0 mL sample of 0.150 M hydrazoic acid is titrated with a 0.150 M NaOH solution.What is the pH after 26.0 mL of base is added? The 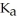 of hydrazoic acid is 1.9 × 10-5.
of hydrazoic acid is 1.9 × 10-5.
A) 2.54
B) 11.47
C) 7.00
D) 4.70
E) 4.74
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q91: Which solution has the greatest buffering capacity?<br>A)0.335
Q92: What change will be caused by addition
Q93: The addition of HF and _ to
Q94: Calculate the pH of a solution that
Q95: Suppose you have just added 500.0 ml
Q97: Of the substances below,_ will decrease the
Q98: The concentration of fluoride ions in a
Q99: Calculate the pH of a solution prepared
Q100: A 25.0 mL sample of 0.723 M
Q101: Why does fluoride treatment render teeth more