Multiple Choice
The pH of a solution prepared by dissolving 0.550 mol of solid methylamine hydrochloride (CH3NH3Cl) in 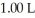 of
of 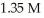 methylamine (CH3NH2) is ________.The Kb for methylamine is
methylamine (CH3NH2) is ________.The Kb for methylamine is 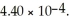 (Assume the final volume is 1.00 L.)
(Assume the final volume is 1.00 L.)
A) 11.03
B) 2.97
C) 3.75
D) 10.64
E) 10.25
Correct Answer:

Verified
Correct Answer:
Verified
Q57: What is the molar solubility of calcium
Q58: 0.78 M Na <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="0.78 M
Q59: The addition of HCl and _ to
Q60: In which aqueous system is <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"
Q61: For any buffer system,the buffer capacity depends
Q63: The addition of KOH and _ to
Q64: Calculate the pH of a solution prepared
Q65: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt=" acid and
Q66: Which one of the following pairs cannot
Q67: The molar solubility of _ is not