Multiple Choice
The pH of a solution prepared by dissolving 0.350 mol of acid in 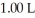 of
of 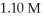 of conjugate base is ________.The Kb for the conjugate base is
of conjugate base is ________.The Kb for the conjugate base is 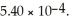 (Assume the final volume is 1.00 L.)
(Assume the final volume is 1.00 L.)
A) 11.23
B) 1.66
C) 11.14
D) 2.77
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q38: The pH of a solution prepared by
Q39: Human blood is considered to be _.<br>A)neutral<br>B)very
Q40: Calculate the maximum concentration (in M)of silver
Q41: Which of the following could be added
Q42: A solution containing which one of the
Q44: A solution is prepared by dissolving 0.23
Q45: A 25.0-mL sample of 0.150 M hypochlorous
Q46: A 50.0 mL sample of a solution
Q47: The solubility of slightly soluble salts containing
Q48: A result of the common-ion effect is