Multiple Choice
Calculate the maximum concentration (in M) of silver ions (Ag+) in a solution that contains 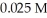 of CO32-.The Ksp of Ag2CO3 is
of CO32-.The Ksp of Ag2CO3 is 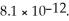
A) 1.8 × 10-5
B) 1.4 × 10-6
C) 2.8 × 10-6
D) 3.2 × 10-10
E) 8.1 × 10-12
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q35: Which solution would have the greatest buffering
Q36: The pH of a solution prepared by
Q37: In which one of the following solutions
Q38: The pH of a solution prepared by
Q39: Human blood is considered to be _.<br>A)neutral<br>B)very
Q41: Which of the following could be added
Q42: A solution containing which one of the
Q43: The pH of a solution prepared by
Q44: A solution is prepared by dissolving 0.23
Q45: A 25.0-mL sample of 0.150 M hypochlorous