Multiple Choice
The mean free path of an oxygen molecule is 2.0 × 10-5 m,when the gas is at a pressure of 120 Pa and a temperature of 275 K.The molecular mass of oxygen is 32.0 g/mol and the Boltzmann constant is 1.38 × 10-23 J/K,Avogadro's number is 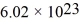 molecules/mole,and the ideal gas constant is
molecules/mole,and the ideal gas constant is 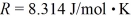 =
= 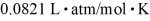 .Assuming that the molecules are moving at their root-mean-square speeds,the average time interval between collisions of an oxygen molecule is closest to
.Assuming that the molecules are moving at their root-mean-square speeds,the average time interval between collisions of an oxygen molecule is closest to
A) 20 ns.
B) 43 ns.
C) 95 ns.
D) 200 ns.
E) 420 ns.
Correct Answer:

Verified
Correct Answer:
Verified
Q18: A 3.2-L volume of neon gas (Ne)is
Q19: 3.00 moles of an ideal gas at
Q20: A cubic box with sides of 20.0
Q21: An ideal gas is kept in a
Q22: A fixed amount of ideal gas is
Q24: What is the mass density of argon
Q25: A sealed 26- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg" alt="A sealed
Q26: A 25-L container holds ideal hydrogen (H<sub>2</sub>)gas
Q27: A sample of an ideal gas is
Q28: The number of molecules in one mole