Short Answer
The figure shows the pV diagram for a certain thermodynamic process.In this process, 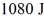 of heat flows into a system,and at the same time the system expands against a constant external pressure of
of heat flows into a system,and at the same time the system expands against a constant external pressure of 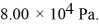 If the volume of the system increases from
If the volume of the system increases from 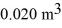 to
to 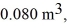 calculate the change in internal (thermal)energy of the system.If the internal (thermal)energy change is nonzero,be sure to indicate whether this energy change is positive or negative.
calculate the change in internal (thermal)energy of the system.If the internal (thermal)energy change is nonzero,be sure to indicate whether this energy change is positive or negative. 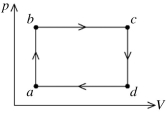
Correct Answer:

Verified
Correct Answer:
Verified
Q24: When an ideal gas increases in volume
Q25: The figure (not to scale)shows a pV
Q26: An ideal gas is allowed to expand
Q27: A container with rigid walls is filled
Q28: When a gas undergoes an isothermal process,there
Q30: The figure shows a pV diagram for
Q31: A certain amount of ideal monatomic gas
Q32: An adiabatic compression is performed on an
Q33: The process shown in the T-V diagram
Q34: An ideal gas is compressed in a