Short Answer
In a thermodynamic process involving 7.8 moles of an ideal gas,the gas is at an initial temperature of 24°C and has an initial volume of 0.040 m3.The gas expands adiabatically to a volume of 0.080 m3.For this gas,CV = 12.27 J/mol • K,and the ideal gas constant is 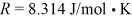 .Calculate the work done by the gas during this expansion.
.Calculate the work done by the gas during this expansion.
Correct Answer:

Verified
Correct Answer:
Verified
Q11: A system has a heat source supplying
Q12: A compression,at a constant pressure of 190
Q13: A fixed amount of ideal gas goes
Q14: During an isothermal process,5.0 J of heat
Q15: When a fixed amount of ideal gas
Q17: A monatomic ideal gas undergoes an isothermal
Q18: An ideal gas increases in temperature from
Q19: 3.0 moles of an ideal gas with
Q20: A cylinder contains 1.2 moles of ideal
Q21: A cylinder contains 24.0 moles of an