Essay
A cylinder contains 1.2 moles of ideal gas,initially at a temperature of 116°C.The cylinder is provided with a frictionless piston,which maintains a constant pressure of 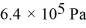 on the gas.The gas is cooled until its temperature has decreased to
on the gas.The gas is cooled until its temperature has decreased to 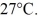 For the gas
For the gas 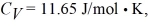 and the ideal gas constant
and the ideal gas constant 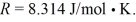 (a)Find the work done by (or on)the gas during this process.Is the work done by or on the gas?
(a)Find the work done by (or on)the gas during this process.Is the work done by or on the gas?
(b)What is the change in the internal (thermal)energy of the gas during this process?
(c)How much heat is transferred to (or from)the gas during this process? Does this heat flow into or out of the gas?
Correct Answer:

Verified
(a)W = -890 J (the negative si...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q15: When a fixed amount of ideal gas
Q16: In a thermodynamic process involving 7.8 moles
Q17: A monatomic ideal gas undergoes an isothermal
Q18: An ideal gas increases in temperature from
Q19: 3.0 moles of an ideal gas with
Q21: A cylinder contains 24.0 moles of an
Q22: The figure shows a pV diagram for
Q23: The process shown in the pV diagram
Q24: When an ideal gas increases in volume
Q25: The figure (not to scale)shows a pV