Multiple Choice
A monatomic ideal gas undergoes an isothermal expansion at 300 K,as the volume increased from 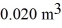 to
to 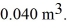 The final pressure is
The final pressure is 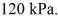 The ideal gas constant is
The ideal gas constant is 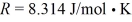 .The heat transfer to the gas is closest to
.The heat transfer to the gas is closest to
A) 3.3 kJ.
B) 1.7 kJ.
C) -3.3 kJ.
D) -1.7 kJ.
E) 0.00 kJ.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: A compression,at a constant pressure of 190
Q13: A fixed amount of ideal gas goes
Q14: During an isothermal process,5.0 J of heat
Q15: When a fixed amount of ideal gas
Q16: In a thermodynamic process involving 7.8 moles
Q18: An ideal gas increases in temperature from
Q19: 3.0 moles of an ideal gas with
Q20: A cylinder contains 1.2 moles of ideal
Q21: A cylinder contains 24.0 moles of an
Q22: The figure shows a pV diagram for