Multiple Choice
What is the highest temperature that the substance shown in the following phase diagram can exist as a liquid? 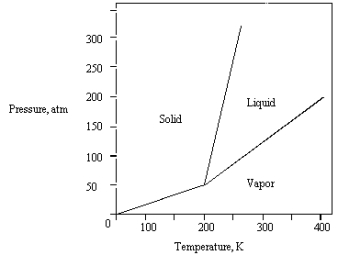
A) any temperature above 200 K
B) 200 K
C) 250 K
D) 350 K
E) 400 K
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q25: Because a solute increases the entropy of
Q35: Consider the phase diagrams for water and
Q67: The vapor pressure of benzene at
Q68: The phase diagram for a pure substance
Q70: The phase diagram for sulfur is given
Q71: the van't Hoff i of HBr,HCl,and HF
Q74: the van't Hoff i of HF differs
Q77: Of the following five materials,which has the
Q85: Calculate the molality of perchloric acid in
Q90: A plot of ln(vapor pressure) versus 1/T