Multiple Choice
The phase diagram for sulfur is given below. 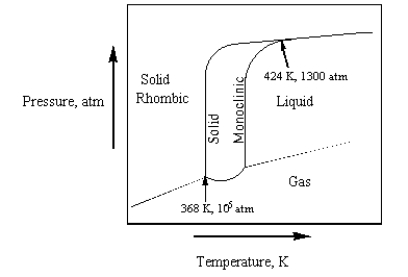 The number of phases that sulfur can exist in and the number of triple points,respectively,are
The number of phases that sulfur can exist in and the number of triple points,respectively,are
A) 3 and 2.
B) 3 and 3.
C) 4 and 2.
D) 3 and 1.
E) 4 and 3.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q25: Because a solute increases the entropy of
Q66: The phase diagram for a pure substance
Q67: The vapor pressure of benzene at
Q68: The phase diagram for a pure substance
Q71: the van't Hoff i of HBr,HCl,and HF
Q72: What is the highest temperature that the
Q74: the van't Hoff i of HF differs
Q85: Calculate the molality of perchloric acid in
Q90: A plot of ln(vapor pressure) versus 1/T
Q165: Which of the following pairs have a