Multiple Choice
The phase diagram for a pure substance is given below. 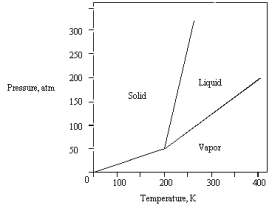 What pressure must be applied to liquefy a sample at 425 K?
What pressure must be applied to liquefy a sample at 425 K?
A) 350 atm
B) The sample cannot be liquefied at 425 K.
C) 150 atm
D) 50 atm
E) 250 atm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: The solubility of oxygen at a certain
Q63: The phase diagram for a pure compound
Q66: The phase diagram for a pure substance
Q67: The vapor pressure of benzene at
Q70: The phase diagram for sulfur is given
Q71: the van't Hoff i of HBr,HCl,and HF
Q72: What is the highest temperature that the
Q85: Calculate the molality of perchloric acid in
Q90: A plot of ln(vapor pressure) versus 1/T
Q165: Which of the following pairs have a