Multiple Choice
Write a balanced equation for the reaction that occurs when hydrogen sulfate ion behaves as a Brønsted-Lowry acid in water.
A) HSO4-(aq) + H2O( 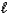 )
) 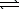 H2SO4(aq) + H3O+(aq)
H2SO4(aq) + H3O+(aq)
B) SO42-(aq) + H2O( 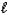 )
) 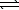 HSO4-(aq) + OH-(aq)
HSO4-(aq) + OH-(aq)
C) HSO4-(aq) + H2O( 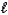 )
) 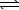 SO42-(aq) + H3O+(aq)
SO42-(aq) + H3O+(aq)
D) HSO4-(aq) + H2O( 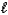 )
) 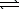 H2SO4(aq) + OH-(aq)
H2SO4(aq) + OH-(aq)
E) H2SO4(aq) + H2O( 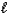 )
) 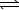 HSO4-(aq) + H3O+(aq)
HSO4-(aq) + H3O+(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q21: Write a balanced net ionic equation
Q22: Which of the following would
Q23: Which of the following reactions best
Q24: What is the smallest whole number
Q25: Which of the following ions is
Q28: Write a balanced chemical equation for
Q29: Which of the following is a
Q30: Give the name of an acidic oxide
Q30: If an aqueous solution of _ is
Q31: Nitroglycerin decomposes violently according to the