Multiple Choice
Which of the following is not a valid resonance structure for N3-?
A) 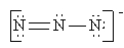
B) 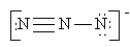
C) 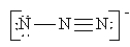
D) 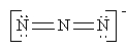
E) all are correct
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: What is the total number of electrons
Q4: Which of the following exhibits resonance?<br>A) SCl<sub>6</sub><br>B)
Q5: Based on electron geometries,which of the following
Q7: In PCl<sub>5</sub>,the Cl-P-Cl bond angle between an
Q9: What is the correct Lewis structure for
Q10: Three non-equivalent Lewis structures for carbonyl
Q11: Using the following data reactions:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt="Using
Q12: How many valence electrons are present in
Q13: Which of the following is a correct
Q42: In benzene,C<sub>6</sub>H<sub>6</sub>,the six carbon atoms are arranged