Multiple Choice
Using the following data reactions: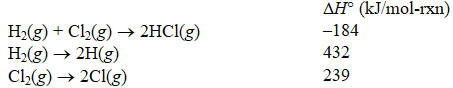
Calculate the energy of an H-Cl bond.
A) 92 kJ/mol
B) 855 kJ/mol
C) 487 kJ/mol
D) 428 kJ/mol
E) 244 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: In PCl<sub>5</sub>,the Cl-P-Cl bond angle between an
Q8: Which of the following is not a
Q9: What is the correct Lewis structure for
Q10: Three non-equivalent Lewis structures for carbonyl
Q12: How many valence electrons are present in
Q13: Which of the following is a correct
Q15: Which of the following statements is/are CORRECT?<br>1)Ionic
Q32: Which of the following molecules or ions
Q39: Electronegativity is a measure of _.<br>A) the
Q42: In benzene,C<sub>6</sub>H<sub>6</sub>,the six carbon atoms are arranged