Multiple Choice
Three non-equivalent Lewis structures for carbonyl sulfide,SCO,are given below.Use the concepts of formal charge and electronegativity to choose the structure that is the best representation. 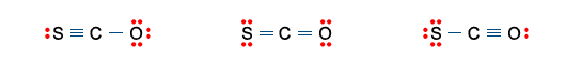
A B C
A) Structure A,because all the formal charges equal 0.
B) Structure B,because all the formal charges equal 0.
C) Structure C,because all the formal charges equal 0.
D) Structure A,because the negative formal charge resides on the most electronegative atom.
E) Structure C,because the negative formal charge resides on the most electronegative atom.
Correct Answer:

Verified
Correct Answer:
Verified
Q5: Based on electron geometries,which of the following
Q7: In PCl<sub>5</sub>,the Cl-P-Cl bond angle between an
Q8: Which of the following is not a
Q9: What is the correct Lewis structure for
Q11: Using the following data reactions:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt="Using
Q12: How many valence electrons are present in
Q13: Which of the following is a correct
Q15: Which of the following statements is/are CORRECT?<br>1)Ionic
Q39: Electronegativity is a measure of _.<br>A) the
Q42: In benzene,C<sub>6</sub>H<sub>6</sub>,the six carbon atoms are arranged