Multiple Choice
Which expression correctly describes the equilibrium constant Kc for the following reaction?
A) 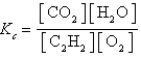
B) 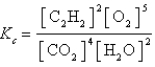
C) 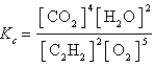
D) 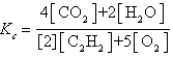
E) 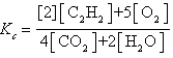
Correct Answer:

Verified
Correct Answer:
Verified
Q10: If the reaction quotient,Q,is equal to K
Q32: Which of the following equilibria would not
Q34: For the equilibrium PCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt="For
Q35: For the reaction TlSCN(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q36: For the equilibrium PCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q38: A flask contains the following chemical system
Q39: At a given temperature,0.0664 mol N<sub>2</sub>O<sub>4</sub>(g)is
Q40: Consider the following equilibrium:<br>CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q41: Exactly 1.0 mol N<sub>2</sub>O<sub>4</sub> is placed in
Q42: Assume that the following chemical reaction