Multiple Choice
A flask contains the following chemical system at equilibrium.
CuCO3(s) 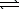 Cu2+(aq) + 2 CO32-(aq)
Cu2+(aq) + 2 CO32-(aq)
Addition of which of the following substances will increase the solubility of CuCO3(s) in water?
1) aqueous hydrochloric acid
2) aqueous sodium carbonate
3) solid copper(II) carbonate
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
Correct Answer:

Verified
Correct Answer:
Verified
Q10: If the reaction quotient,Q,is equal to K
Q34: For the equilibrium PCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt="For
Q35: For the reaction TlSCN(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q36: For the equilibrium PCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q37: Which expression correctly describes the equilibrium constant
Q39: At a given temperature,0.0664 mol N<sub>2</sub>O<sub>4</sub>(g)is
Q40: Consider the following equilibrium:<br>CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q41: Exactly 1.0 mol N<sub>2</sub>O<sub>4</sub> is placed in
Q42: Assume that the following chemical reaction
Q43: What is the reaction quotient,Q,for the equilibrium<br><img