Multiple Choice
Assume that the following chemical reaction is at equilibrium.
2 ICl(g) 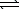 I2(g) + Cl2(g) H = +26.9 kJ
I2(g) + Cl2(g) H = +26.9 kJ
At 25 C,Kp = 2.0 105.If the temperature is increase to 45 C,which statement applies?
A) Kp will decrease and the reaction will proceed in the backward direction.
B) Kp will decrease and the reaction will proceed in the forward direction.
C) Kp will remain unchanged and the reaction will proceed in the forward direction.
D) Kp will increase and the reaction will proceed in the backward direction.
E) Kp will increase and the reaction will proceed in the forward direction.
Correct Answer:

Verified
Correct Answer:
Verified
Q37: Which expression correctly describes the equilibrium constant
Q38: A flask contains the following chemical system
Q39: At a given temperature,0.0664 mol N<sub>2</sub>O<sub>4</sub>(g)is
Q40: Consider the following equilibrium:<br>CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q41: Exactly 1.0 mol N<sub>2</sub>O<sub>4</sub> is placed in
Q43: What is the reaction quotient,Q,for the equilibrium<br><img
Q44: When gaseous carbon monoxide and hydrogen are
Q45: In an experiment,0.42 mol H<sub>2</sub> and 0.42
Q46: At 25 <sup> <span class="ql-formula" data-value="\circ"><span
Q47: The reaction quotient,Q,for a system is <img