Multiple Choice
Write a balanced chemical equation for the oxidation of Cd(s) by concentrated nitric acid,producing NO2(g) and Cd2+(aq) .
A) HNO3(aq) + Cd(s) Cd2+(aq) + NO2(g) + OH-(aq)
B) 2 HNO3(aq) + Cd(s) Cd2+(aq) + 2 NO2(g) + 2 OH-(aq)
C) HNO3(aq) + Cd(s) + H+(aq) Cd2+(aq) + NO2(g) + H2O( 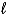 )
)
D) 4 HNO3(aq) + Cd(s) Cd2+(aq) + 2 NO2(g) + 2H2O( 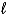 ) + 2 NO3-(aq)
) + 2 NO3-(aq)
E) HNO3(aq) + Cd(s) Cd2+(aq) + NO2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q24: Assuming the following reaction proceeds in
Q25: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q26: The following reaction occurs spontaneously.<br>2 H<sup>+</sup>(aq)+
Q27: When an aqueous solution of sodium
Q28: Which of the following statements is
Q30: One kind of battery used in
Q31: The electrochemical reaction which powers a
Q33: Balance the following half-reaction occurring in
Q34: Consider the following half-reactions:<br>Ag<sup>+</sup>(aq)+ e<sup>-</sup>
Q51: In an electrolytic cell,reduction occurs at the