Multiple Choice
When an aqueous solution of sodium sulfate is electrolyzed,what are the expected products?
Reduction Half-Reaction E° (V) 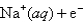 Na(s) -2.71
Na(s) -2.71
2H2O(l) + 2e- H2(g) + 2OH-(aq) -0.83
2H+(aq) + 2e- H2(g) 0.00
O2(g) + 4H+(aq) + 4e- 2H2O(l) 1.23
S2O82-(aq) + 2e- 2SO42-(aq) 2.01
A) Na(s) and H2(g)
B) H2(g) ,OH-(aq) ,O2(g) ,and H+(aq)
C) O2(g) ,H+(aq) ,and Na(s)
D) H2(g) ,OH-(aq) ,and Na(s)
E) H2(g) ,OH-(aq) ,and S2O82-(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q22: The fuel cells used aboard NASA's
Q23: Write a balanced chemical equation for
Q24: Assuming the following reaction proceeds in
Q25: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q26: The following reaction occurs spontaneously.<br>2 H<sup>+</sup>(aq)+
Q28: Which of the following statements is
Q29: Write a balanced chemical equation for
Q30: One kind of battery used in
Q31: The electrochemical reaction which powers a
Q51: In an electrolytic cell,reduction occurs at the