Multiple Choice
One kind of battery used in watches contains mercury(II) oxide.As current flows,the mercury(II) oxide is reduced to mercury.
HgO(s) + H2O( 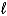 ) + 2 e- Hg(
) + 2 e- Hg( 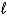 ) + 2 OH-(aq)
) + 2 OH-(aq)
If 2.3 10-5 amperes flows continuously for 1200 days,what mass of Hg( 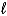
) is produced?
A) 2.5 g
B) 5.0 g
C) 9.9 g
D) 13 g
E) 15 g
Correct Answer:

Verified
Correct Answer:
Verified
Q25: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q26: The following reaction occurs spontaneously.<br>2 H<sup>+</sup>(aq)+
Q27: When an aqueous solution of sodium
Q28: Which of the following statements is
Q29: Write a balanced chemical equation for
Q31: The electrochemical reaction which powers a
Q33: Balance the following half-reaction occurring in
Q34: Consider the following half-reactions:<br>Ag<sup>+</sup>(aq)+ e<sup>-</sup>
Q35: Write a balanced chemical equation for
Q51: In an electrolytic cell,reduction occurs at the