Multiple Choice
Which of the following structures contains an sp2-hybridized oxygen atom because a lone pair on oxygen is delocalized via resonance? 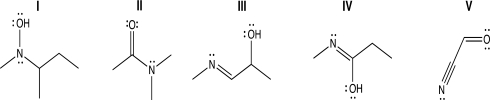
A) Structure I
B) Structure II
C) Structures II and V
D) Structure IV
E) Structures I and IV
Correct Answer:

Verified
Correct Answer:
Verified
Q45: What is the geometry and hybridization of
Q46: According to valence bond theory,which atomic orbitals
Q47: Naltrexone is an antagonist at the mu
Q48: What abbreviation is used to designate the
Q49: Using line structures,deduce individual resonance contributors from
Q51: Bioymifi is a novel small molecule that
Q52: Which C<font face="symbol"></font><font face="symbol"></font><font face="symbol"></font>C bond in
Q53: Use your knowledge of hybridization to fill
Q54: Rank the highlighted σ bonds in order
Q55: For one or more of the following