Short Answer
Using line structures,deduce individual resonance contributors from the resonance hybrid structure given here.Identify any lone pairs that are localized,rather than delocalized.Based on orbital hybridization theory,what orbitals accommodate these lone pairs? 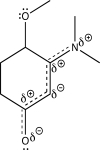
Correct Answer:

Verified
 The sp3 oxygen of the ether us...
The sp3 oxygen of the ether us...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q44: Acetonitrile,C<sub>2</sub>H<sub>3</sub>N,is a polar aprotic solvent commonly used
Q45: What is the geometry and hybridization of
Q46: According to valence bond theory,which atomic orbitals
Q47: Naltrexone is an antagonist at the mu
Q48: What abbreviation is used to designate the
Q50: Which of the following structures contains an
Q51: Bioymifi is a novel small molecule that
Q52: Which C<font face="symbol"></font><font face="symbol"></font><font face="symbol"></font>C bond in
Q53: Use your knowledge of hybridization to fill
Q54: Rank the highlighted σ bonds in order