Multiple Choice
What is the geometry and hybridization of the central carbon atom in the cumulene below? 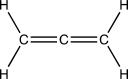
A) sp3 hybridized and tetrahedral
B) sp3 hybridized and trigonal pyramidal
C) sp2 hybridized and trigonal planar
D) sp2 hybridized and bent
E) sp hybridized and linear
Correct Answer:

Verified
Correct Answer:
Verified
Q40: Draw line structures of a molecule with
Q41: An alkene contains a double bond.Why is
Q42: Which of the following statements about molecular
Q43: From left to right,identify the hybridization of
Q44: Acetonitrile,C<sub>2</sub>H<sub>3</sub>N,is a polar aprotic solvent commonly used
Q46: According to valence bond theory,which atomic orbitals
Q47: Naltrexone is an antagonist at the mu
Q48: What abbreviation is used to designate the
Q49: Using line structures,deduce individual resonance contributors from
Q50: Which of the following structures contains an