Multiple Choice
Diatomic O2 can react with the element magnesium to form magnesium oxide (MgO) .The balanced chemical equation is: 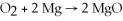
If 4 moles of magnesium totally reacted with more than enough O2,how many moles of MgO would be expected to form?
A) 1 mole
B) 2 moles
C) 4 moles
D) 8 moles
E) not enough information
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Calculate the maximum number of grams of
Q14: For the following reaction you have 8
Q17: If the theoretical yield of the reaction
Q18: How many grams of the excess reactant
Q37: How many grams of NO<sub>2</sub> are theoretically
Q38: Determine the theoretical yield of C when
Q47: Which ingredient is the limiting reactant if
Q76: The enthalpy of reaction,△H <sub>rxn</sub>,is the amount
Q80: How many grams of water are made
Q86: If the theoretical yield of a reaction