Multiple Choice
Calculate the maximum number of grams of NH3 that can be produced by the reaction of 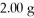
Of N2 with 3.00 g H2.
Reaction: N2(g) + 3 H2(g) → 2 NH3(g)
A) 0.964
B) 2.43
C) 4.00
D) 17.0
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: Diatomic O<sub>2</sub> can react with the element
Q17: If the theoretical yield of the reaction
Q32: The theoretical yield of a reaction is
Q38: Determine the theoretical yield of C when
Q74: The primary source for the rising carbon
Q76: The enthalpy of reaction,△H <sub>rxn</sub>,is the amount
Q80: How many grams of water are made
Q85: How many moles of sodium metal are
Q86: If the theoretical yield of a reaction
Q96: Which of the following is TRUE?<br>A)Stoichiometry shows