Multiple Choice
If the theoretical yield of the reaction below corresponds to 25.3 g and the percent yield of the reaction is known to be reproducibly 81.1%,calculate the actual yield.
Given: 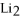
O + 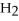
O → 2 LiOH
A) 20.5 g
B) 48.9 g
C) 45.8 g
D) 81.1 g
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Calculate the maximum number of grams of
Q14: For the following reaction you have 8
Q16: Diatomic O<sub>2</sub> can react with the element
Q18: How many grams of the excess reactant
Q37: How many grams of NO<sub>2</sub> are theoretically
Q38: Determine the theoretical yield of C when
Q47: Which ingredient is the limiting reactant if
Q61: How many waffles can be made from
Q76: The enthalpy of reaction,△H <sub>rxn</sub>,is the amount
Q86: If the theoretical yield of a reaction