Multiple Choice
A balanced chemical equation used to prepare ammonium carbonate, (NH4) 2CO3 ,is: 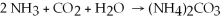
Which choice of reactant quantities shown below would result in the greatest amount of ammonium carbonate being formed?
A) React 2 moles NH3 ,1 mole CO2 ,and 1 mole H2O
B) React 2 moles NH3 ,8 moles CO2 ,and 8 moles H2O
C) React 4 moles NH3 ,1 mole CO2 ,and 2 moles H2O
D) React 4 moles NH3 ,2 moles CO2 ,and 2 moles H2O
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q26: The conversion factor for moles of carbon
Q45: The limiting reactant is the product that
Q57: How many moles of lithium nitrate are
Q62: What is the theoretical yield of waffles
Q79: What is the limiting reactant for the
Q92: Starting with 156 g <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6109/.jpg" alt="Starting
Q98: What is the excess reactant for the
Q100: Thermal energy flows into the reaction and
Q101: How many grams of water are needed
Q102: Given the chemical equation: 2 Ca +