Multiple Choice
How many kJ of heat are needed to completely melt 17.3 g of 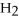
O,given that the water is at its melting point? The heat of fusion for water is 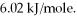
A) 0.961
B) 5.79
C) 1.04
D) 6.26
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q18: The change of a substance from a
Q22: Dry ice (solid C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6109/.jpg" alt="Dry
Q26: Which intermolecular forces are found in <img
Q30: A 250 gram sample of water at
Q31: Which noble gas has the highest boiling
Q32: Which substance below has dipole-dipole forces?<br>A)CH<sub>4</sub><br>B)CO<sub>2</sub><br>C)F<sub>2</sub><br>D)H<sub>2</sub>S<br>E)none of
Q53: Assuming that the molecules carbon monoxide (CO)and
Q76: The reason for many of the unique
Q82: A situation where two opposite processes are
Q106: What happens as you start to add