Multiple Choice
Refer to the figure below. 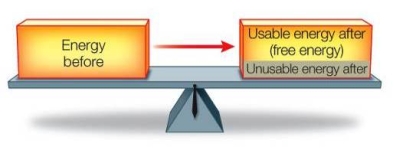 Does this figure illustrate both the first and second laws of thermodynamics?
Does this figure illustrate both the first and second laws of thermodynamics?
A) No, the figure illustrates the first law of thermodynamics only, because it shows how the amount of energy remains constant during a transformation.
B) No, the figure illustrates the second law of thermodynamics only, because it shows how the amount of usable energy decreases as the result of a transformation.
C) No, the figure illustrates the second law of thermodynamics only, because both laws cannot operate simultaneously in the same system.
D) Yes, the figure illustrates both laws of thermodynamics because both laws state the same basic principle.
E) Yes, the figure illustrates both laws of thermodynamics because it shows conservation of total energy and a decrease in usable energy during a transformation.
Correct Answer:

Verified
Correct Answer:
Verified
Q67: Refer to the graph below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q68: A particular enzyme is used to convert
Q69: A chemical cross-linking reagent has two reactive
Q70: Free energy is<br>A) enthalpy.<br>B) entropy.<br>C) usable energy.<br>D)
Q71: The phosphorylation of glucose to glucose 6-phosphate
Q73: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q74: Refer to the graphs below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q75: Refer to the table below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q76: Which of the following can participate in
Q77: Two amino acids shown to be essential