Multiple Choice
Refer to the figure below. 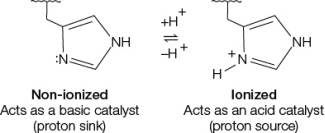 The figure shows how a histidine group at the active site of an enzyme could function either as a base (left) or as an acid (right) .The pKa of histidine is 6.0, which means that at pH 6.0, there are equal numbers of ionized and nonionized histidine groups since a pKa is an acid dissociation constant.With this in mind, what would you expect about the pH sensitivity of enzyme X that uses histidine as a base catalyst compared with enzyme Y that uses histidine as an acid catalyst?
The figure shows how a histidine group at the active site of an enzyme could function either as a base (left) or as an acid (right) .The pKa of histidine is 6.0, which means that at pH 6.0, there are equal numbers of ionized and nonionized histidine groups since a pKa is an acid dissociation constant.With this in mind, what would you expect about the pH sensitivity of enzyme X that uses histidine as a base catalyst compared with enzyme Y that uses histidine as an acid catalyst?
A) Enzyme X and enzyme Y would both have the highest activities at pH 6.0, with lower activities at pH values above and below pH 6.0.
B) Enzyme X and enzyme Y would both have lower activities at pH values below 6.0 and higher activities at pH values above 6.0.
C) Enzyme X and enzyme Y would both have higher activities at pH values below 6.0 and lower activities at pH values above 6.0.
D) Enzyme X would have higher activities below pH 6.0 and lower activities above pH 6.0, while enzyme Y would have lower activities below pH 6.0 and higher activities above pH 6.0.
E) Enzyme X would have lower activities below pH 6.0 and higher activities above pH 6.0, while enzyme Y would have higher activities below pH 6.0 and lower activities above pH 6.0.
Correct Answer:

Verified
Correct Answer:
Verified
Q68: A particular enzyme is used to convert
Q69: A chemical cross-linking reagent has two reactive
Q70: Free energy is<br>A) enthalpy.<br>B) entropy.<br>C) usable energy.<br>D)
Q71: The phosphorylation of glucose to glucose 6-phosphate
Q72: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q74: Refer to the graphs below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q75: Refer to the table below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q76: Which of the following can participate in
Q77: Two amino acids shown to be essential
Q78: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"